Two electrolytic cells containing 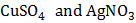 respectively are connected in series and a current is passed through them until 2 mg of copper is deposited in the first cell. The amount of silver deposited in the second cell during this time in approximately (atomic weight of copper and silver are 63.6 and 108.0 )
respectively are connected in series and a current is passed through them until 2 mg of copper is deposited in the first cell. The amount of silver deposited in the second cell during this time in approximately (atomic weight of copper and silver are 63.6 and 108.0 )
a)
1.7 mg
b)
3.4 mg
c)
5.1 mg
d)
6.8 mg
Two electrolytic cells containing 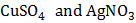 respectively are connected in series and a current is passed through them until 2 mg of copper is deposited in the first cell. The amount of silver deposited in the second cell during this time in approximately (atomic weight of copper and silver are 63.6 and 108.0 )
respectively are connected in series and a current is passed through them until 2 mg of copper is deposited in the first cell. The amount of silver deposited in the second cell during this time in approximately (atomic weight of copper and silver are 63.6 and 108.0 )
a)
1.7 mg
b)
3.4 mg
c)
5.1 mg
d)
6.8 mg
Question ID - 150229 | SaraNextGen Answer
Two electrolytic cells containing 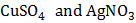 respectively are connected in series and a current is passed through them until 2 mg of copper is deposited in the first cell. The amount of silver deposited in the second cell during this time in approximately (atomic weight of copper and silver are 63.6 and 108.0 )
respectively are connected in series and a current is passed through them until 2 mg of copper is deposited in the first cell. The amount of silver deposited in the second cell during this time in approximately (atomic weight of copper and silver are 63.6 and 108.0 )
a)
1.7 mg
b)
3.4 mg
c)
5.1 mg
d)
6.8 mg
|
Two electrolytic cells containing |
|||||||
|
a) |
1.7 mg |
b) |
3.4 mg |
c) |
5.1 mg |
d) |
6.8 mg |
1 Answer - 5876 Votes

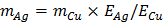
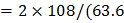 /2) = 6.8
/2) = 6.8 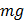 .
.