According to Faraday’s law of electrolysis, the amount of decomposition is proportional to
a)
1/time for which current passes
b)
Electrochemical equivalent of the substance
c)
1/current
d)
1/electrochemical equivalent
According to Faraday’s law of electrolysis, the amount of decomposition is proportional to
a)
1/time for which current passes
b)
Electrochemical equivalent of the substance
c)
1/current
d)
1/electrochemical equivalent
Question ID - 150240 | SaraNextGen Answer
According to Faraday’s law of electrolysis, the amount of decomposition is proportional to
a)
1/time for which current passes
b)
Electrochemical equivalent of the substance
c)
1/current
d)
1/electrochemical equivalent
|
According to Faraday’s law of electrolysis, the amount of decomposition is proportional to |
|||
|
a) |
1/time for which current passes |
b) |
Electrochemical equivalent of the substance |
|
c) |
1/current |
d) |
1/electrochemical equivalent |
1 Answer - 5876 Votes
3537
Answer Key : (b) -
Answer Link|
(b) The amount of decomposition ( |

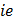 , mass of the substance liberated during electrolysis) is proportional to ECE of the substance.
, mass of the substance liberated during electrolysis) is proportional to ECE of the substance.