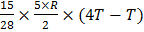A 15 g mass of nitrogen gas is enclosed in a vessel at temperature 270 C. Amount of heat transferred to the gas, so that rms velocity of molecules is doubled, is about
|
a. |
10 kJ |
b. |
0.9 kJ |
|
c. |
6 kJ |
d. |
14 kJ |
Question ID - 50116 | SaraNextGen Answer A 15 g mass of nitrogen gas is enclosed in a vessel at temperature 270 C. Amount of heat transferred to the gas, so that rms velocity of molecules is doubled, is about
a.
10 kJ
b.
0.9 kJ
c.
6 kJ
d.
14 kJ
A 15 g mass of nitrogen gas is enclosed in a vessel at temperature 270 C. Amount of heat transferred to the gas, so that rms velocity of molecules is doubled, is about
|
a. |
10 kJ |
b. |
0.9 kJ |
|
c. |
6 kJ |
d. |
14 kJ |
1 Answer - 5876 Votes

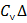 T as gas in closed vessel
T as gas in closed vessel