A hydrogen atom, initially in the ground state is excited by absorbing a photon of wavelength 980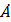 . The radius of the atom in the excited state, it terms of Bohr radius a0, will be: (hc= 12500 eV
. The radius of the atom in the excited state, it terms of Bohr radius a0, will be: (hc= 12500 eV 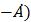
(a)
9a0
(b)
25a0
(c)
4a0
(d)
16a0
A hydrogen atom, initially in the ground state is excited by absorbing a photon of wavelength 980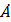 . The radius of the atom in the excited state, it terms of Bohr radius a0, will be: (hc= 12500 eV
. The radius of the atom in the excited state, it terms of Bohr radius a0, will be: (hc= 12500 eV 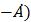
(a)
9a0
(b)
25a0
(c)
4a0
(d)
16a0
Question ID - 50290 | SaraNextGen Answer
A hydrogen atom, initially in the ground state is excited by absorbing a photon of wavelength 980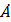 . The radius of the atom in the excited state, it terms of Bohr radius a0, will be: (hc= 12500 eV
. The radius of the atom in the excited state, it terms of Bohr radius a0, will be: (hc= 12500 eV 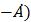
(a)
9a0
(b)
25a0
(c)
4a0
(d)
16a0
|
A hydrogen atom, initially in the ground state is excited by absorbing a photon of wavelength 980 |
|||
|
(a) |
9a0 |
(b) |
25a0 |
|
(c) |
4a0 |
(d) |
16a0 |
1 Answer - 5876 Votes
3537
Answer Key : (d) -
Answer LinkEnergy of photon=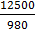 =12.75eV
=12.75eV
∴Electron will excite to n=4 since ‘R’∝ n2
∴Radius of atom will be 16a0
