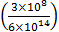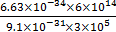If the de-Broglie wavelength of an electron is equal to 10−3 times the wavelength of a photon of frequency 6×1014 Hz, then the speed of electron is equal to : (Speed of light =3 ×108 m/s Planck’s constant=6.63×10−34 J.s Mass of electron =9.1×10−31 kg)
(a)
1.45× m/s
(b)
1.7×106 m/s
(c)
1.8×106 m/s
(d)
1.1 ×106 m/s
If the de-Broglie wavelength of an electron is equal to 10−3 times the wavelength of a photon of frequency 6×1014 Hz, then the speed of electron is equal to : (Speed of light =3 ×108 m/s Planck’s constant=6.63×10−34 J.s Mass of electron =9.1×10−31 kg)
(a)
1.45× m/s
(b)
1.7×106 m/s
(c)
1.8×106 m/s
(d)
1.1 ×106 m/s
Question ID - 50303 | SaraNextGen Answer
If the de-Broglie wavelength of an electron is equal to 10−3 times the wavelength of a photon of frequency 6×1014 Hz, then the speed of electron is equal to : (Speed of light =3 ×108 m/s Planck’s constant=6.63×10−34 J.s Mass of electron =9.1×10−31 kg)
(a)
1.45× m/s
(b)
1.7×106 m/s
(c)
1.8×106 m/s
(d)
1.1 ×106 m/s
|
If the de-Broglie wavelength of an electron is equal to 10−3 times the wavelength of a photon of frequency 6×1014 Hz, then the speed of electron is equal to : (Speed of light =3 ×108 m/s Planck’s constant=6.63×10−34 J.s Mass of electron =9.1×10−31 kg) |
|||
|
(a) |
1.45× m/s |
(b) |
1.7×106 m/s |
|
(c) |
1.8×106 m/s |
(d) |
1.1 ×106 m/s |
1 Answer - 5876 Votes

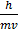 =10-3
=10-3