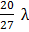In a hydrogen like atom, when an electron jumps from the M-shell to the L-shell, the wavelength of emitted radiation is 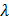 . If an electron jumps from N-shell to the L-shell, the wavelength of emitted radiation will be :
. If an electron jumps from N-shell to the L-shell, the wavelength of emitted radiation will be :
|
a. |
|
b. |
|
|
c. |
|
d. |
|
Question ID - 50369 | SaraNextGen Answer In a hydrogen like atom, when an electron jumps from the M-shell to the L-shell, the wavelength of emitted radiation is 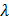 . If an electron jumps from N-shell to the L-shell, the wavelength of emitted radiation will be :
. If an electron jumps from N-shell to the L-shell, the wavelength of emitted radiation will be :
a.
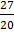
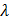
b.
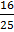
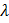
c.
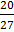
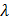
d.
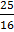
In a hydrogen like atom, when an electron jumps from the M-shell to the L-shell, the wavelength of emitted radiation is 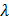 . If an electron jumps from N-shell to the L-shell, the wavelength of emitted radiation will be :
. If an electron jumps from N-shell to the L-shell, the wavelength of emitted radiation will be :
|
a. |
|
b. |
|
|
c. |
|
d. |
|
1 Answer - 5876 Votes

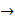 L steel
L steel = K
= K 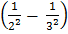 =
= 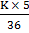
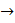 L
L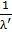 = K
= K 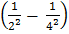 =
= 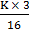
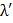 =
=