In a copper voltmeter , if the current (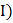 and time
and time 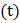 variations of the type as shown in figure, the mass deposited in 30 min is [Atomic weight of copper is 63.5 and Faraday constant is 96500 C per g equivalent]
variations of the type as shown in figure, the mass deposited in 30 min is [Atomic weight of copper is 63.5 and Faraday constant is 96500 C per g equivalent]
a)
0.078 g
b)
0.054 g
c)
0.039 g
d)
0.0195 g
In a copper voltmeter , if the current (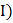 and time
and time 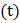 variations of the type as shown in figure, the mass deposited in 30 min is [Atomic weight of copper is 63.5 and Faraday constant is 96500 C per g equivalent]
variations of the type as shown in figure, the mass deposited in 30 min is [Atomic weight of copper is 63.5 and Faraday constant is 96500 C per g equivalent]
a)
0.078 g
b)
0.054 g
c)
0.039 g
d)
0.0195 g
Question ID - 150389 | SaraNextGen Answer
In a copper voltmeter , if the current (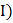 and time
and time 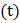 variations of the type as shown in figure, the mass deposited in 30 min is [Atomic weight of copper is 63.5 and Faraday constant is 96500 C per g equivalent]
variations of the type as shown in figure, the mass deposited in 30 min is [Atomic weight of copper is 63.5 and Faraday constant is 96500 C per g equivalent]
a)
0.078 g
b)
0.054 g
c)
0.039 g
d)
0.0195 g
|
In a copper voltmeter , if the current ( |
|||||||
|
a) |
0.078 g |
b) |
0.054 g |
c) |
0.039 g |
d) |
0.0195 g |
1 Answer - 5876 Votes

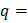 area under graph
area under graph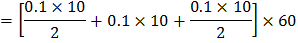
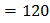 C
C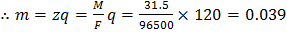 g
g