A mixture of 2 moles of helium gas (atomic mass=4u), and 1 mole of argon gas (atomic mass=40u) is kept at 300 K in a container. The ratio of their rms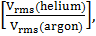 is close to :
is close to :
|
a. |
2.24 |
b. |
0.45 |
|
c. |
0.32 |
d. |
3.16 |
Question ID - 50039 | SaraNextGen Answer A mixture of 2 moles of helium gas (atomic mass=4u), and 1 mole of argon gas (atomic mass=40u) is kept at 300 K in a container. The ratio of their rms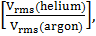 is close to :
is close to :
a.
2.24
b.
0.45
c.
0.32
d.
3.16
A mixture of 2 moles of helium gas (atomic mass=4u), and 1 mole of argon gas (atomic mass=40u) is kept at 300 K in a container. The ratio of their rms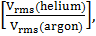 is close to :
is close to :
|
a. |
2.24 |
b. |
0.45 |
|
c. |
0.32 |
d. |
3.16 |
1 Answer - 5876 Votes

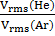 =
=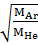 =
=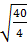 =3.16
=3.16